Abstract
Background. Innovations in pediatric oncology have led to advances that take the place of older, more toxic therapies, making it possible for more children to survive. As this population grows, it is essential to monitor and carefully study late effects. Perhaps most insidious and far reaching in its effect on the daily lives of survivors is neurocognitive dysfunction that impacts school performance, occupational attainment, and quality of life. The populations at greatest risk for neurocognitive dysfunction in the pediatric hematology oncology population include children with central nervous system (CNS) tumors, leukemia (ALL), and sickle cell disease (SCD). What is known about neurocognitive dysfunction in these populations has come from studying these groups separately and with heterogeneous tools, putting each group on a different "measuring stick."
Methods. The current study seeks to quantify and compare the magnitude and type of neurocognitive dysfunction between groups of children with CNS tumors, pre-B ALL, and SCD by employing a common methodology to directly compare these groups to each other. Neurocognitive function was assessed using the NIH Toolbox Cognition Battery. Children between the ages of 7-12 were included without pre-existing neurological disorders. Children with CNS tumors or pre-B ALL were within 18 months of completing therapy. Children with SCD possessed the SS or Sβ0 genotype, regularly took hydroxyurea, had no history of overt stroke, and did not receive chronic transfusions. NIH Toolbox Cognition Battery scores are presented as age-adjusted standard scores with a mean of 100, standard deviation of 15. Effect sizes were calculated using Cohen's d statistic. We hypothesized that these groups would perform significantly differently from normative data and differently from each other, with children with CNS tumors showing the greatest deficits, followed by children with SCD, with children with ALL showing the fewest deficits.
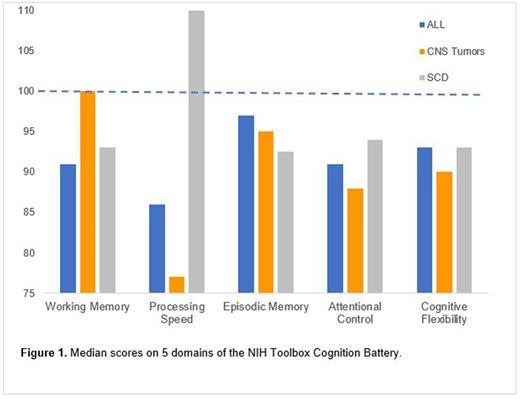
Results. Fifty-three patients were included in analysis (n = 11 CNS tumor, n = 27 ALL, n = 15 SCD). Children with ALL demonstrated a large, negative effect in processing speed (median=86, IQR 65-95; Cohen's d = -0.93) and medium, negative effects in working memory (median=91, IQR 84.5-105.5, d = -0.6) and attentional control (median= 92, IQR 80-102, d = -0.6; Figure 1). Children with CNS tumors experienced medium deficits in cognitive flexibility (median=90, IQR 84-97, d = -0.67) and large, negative effects in processing speed (median=77, IQR 69-95, d = -1.53) and attentional control (median=88, IQR 76.5-93, d = -0.8). Children with SCD demonstrated medium, negative effects in episodic memory (median=92.5, IQR 85-102.8, d = -0.5). For the overall cognitive fluid composite score, children with SCD performed in the average range (median=99.5, IQR 83.2-105), while children diagnosed with malignancies demonstrated poorer performance with large effect sizes (ALL median=87, IQR 82-95, d = -0.87; CNS tumor median=84, IQR 74.5-95.5, d = -1.07).
Conclusions. Preliminary analyses suggest that at-risk groups of children with ALL, CNS tumors, and SCD experience neurocognitive deficits differently from one another in multiple domains. Future studies should employ findings like these to develop and implement disease- and domain-specific interventions to reverse or alleviate neurocognitive deficits.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal